Published June 19, 2025
The medical scientific community thrives on the free flow of clinical insights, the rigorous scrutiny of experimental findings, and the rapid dissemination of knowledge that can lead to improved patient outcomes. Yet, the current medical research publishing process often feels like a clogged artery, hindering the very progress it aims to facilitate. This growing frustration stems from a system often prioritizing profit and prestige over efficiency and accessibility. As engineers, our instinct is to identify bottlenecks and design solutions. It’s time we applied this mindset to the medical research publishing workflow.
The drawn-out peer review processes for critical clinical trial data, the prohibitive costs of accessing vital epidemiological studies, and the disproportionate emphasis on journal impact factors over the inherent scientific and clinical value of the research itself – these are not minor annoyances. They represent systemic inefficiencies that actively stifle innovation in diagnostics and therapeutics, delay the application of crucial discoveries in patient care, and ultimately impact public health outcomes. Just as we engineer streamlined production lines and optimized data pipelines in other complex industries, we can envision and build a more efficient, transparent, and equitable system for sharing medical scientific output.
Inspired by these pressing concerns, and leveraging Medetary’s foundational principles, we propose an engineering-driven response focused on transforming the medical research lifecycle through precision, transparency, and accessibility.
Imagine a team of clinicians, epidemiologists, and basic scientists trying to synthesize data for a critical review, build consensus on clinical guidelines, or draft a multi-center trial protocol. They are often using scattered documents, battling version conflicts, and struggling with real-time feedback across different hospital systems and research institutions. This chaotic scenario mirrors the reality of collaborative medical scientific writing today. Researchers often juggle multiple document versions, navigate conflicting edits, and struggle with real-time feedback loops across different time zones and institutions. This fragmentation leads to wasted time, version control nightmares, and unnecessary friction in the initial stages of research dissemination, delaying the crucial synthesis of medical evidence.
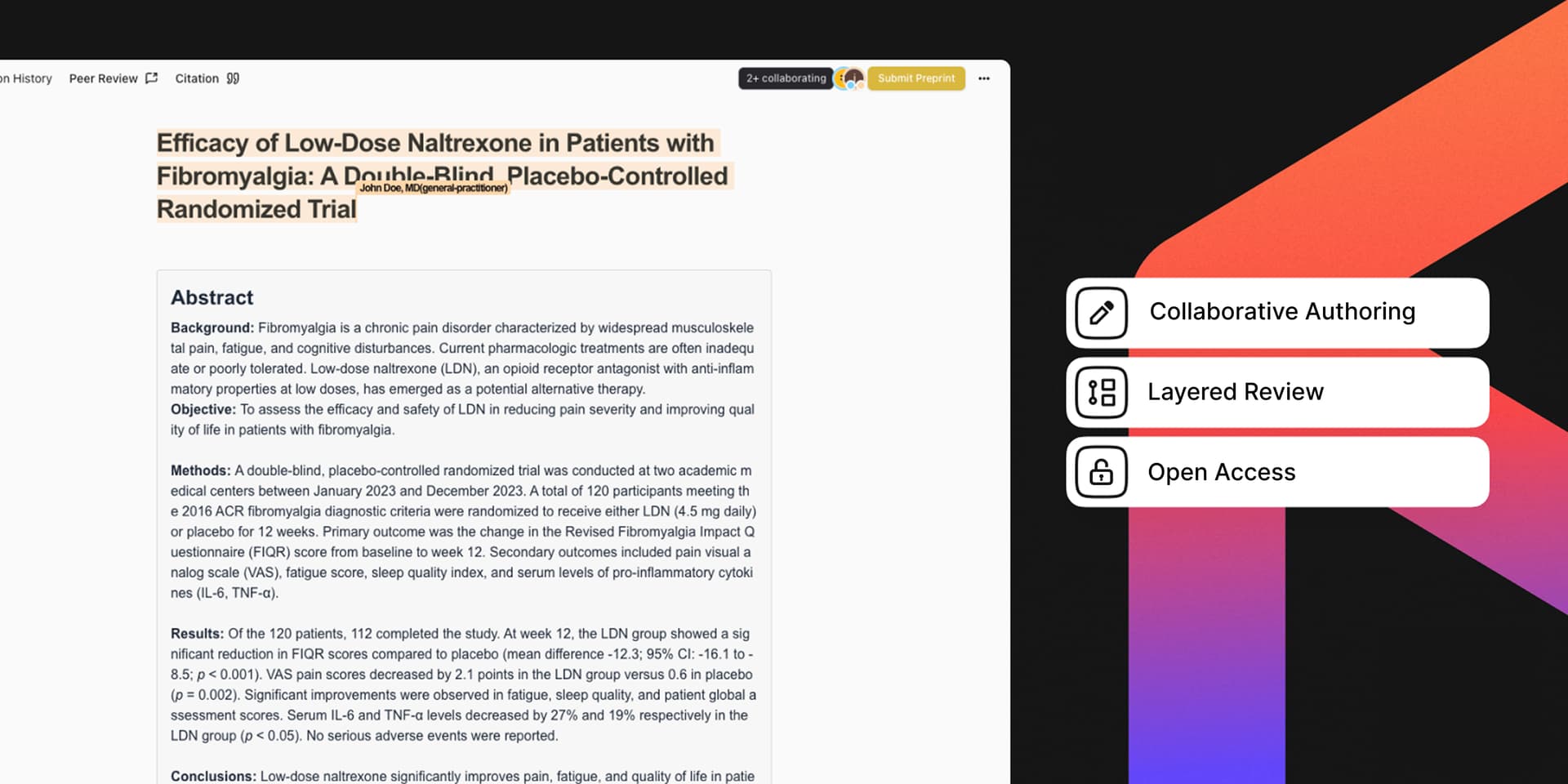
An engineering-centric approach demands a single, unified environment for medical research. Platforms like Medetary are designed precisely for this need. By offering real-time collaborative workspaces with integrated live editing and transparent version tracking, we provide a direct solution tailored for medical documents.
How it works (Technical Mechanisms):
At its core, this involves a robust, distributed data synchronization layer, critically designed with HIPAA and GDPR compliance in mind for sensitive medical data. Medetary can be thought of as Notion plus GitHub for medical publishing. Inspired by the fluid collaboration of platforms like GitHub for code development and Notion for knowledge organization, Medetary's architecture leverages:
Operational Transformation (OT) or Conflict-Free Replicated Data Types (CRDTs): These algorithms enable multiple medical professionals to concurrently edit the same document (be it a study protocol, a meta-analysis, or a grant application) with changes propagating in milliseconds. Each keystroke or alteration from any collaborator is transformed and applied to all other instances, ensuring eventual consistency without manual merging conflicts. This is the bedrock of "live editing" for complex medical texts, much like a Notion document.
Immutable Version Histories (Audit Trails) with Data Provenance: Every discrete change, from a single word edit in a patient consent form section to a figure update in a research manuscript, is recorded as a distinct, timestamped operation. This isn't just about saving document versions; it's about a granular, append-only ledger of every contribution. This audit trail is cryptographically secured, ensuring that past versions cannot be tampered with, providing irrefutable provenance for medical co-authorship and compliance records. The continuous tracking means that every version of a manuscript remains accessible, helping authors and readers understand how feedback shaped the research, mirroring the version control and commit history benefits seen in platforms like GitHub.
Role-Based Access Control (RBAC) at Document & Element Level for Sensitive Information: Beyond simple "read/write" permissions, Medetary employs a finely-tuned RBAC system adapted for medical research sensitivities. This allows principal investigators and data stewards to define permissions not just for entire documents, but for specific sections of a clinical trial report, individual data tables, or even specific comments on patient de-identification methods. This ensures "seamless global co-authorship" while maintaining stringent control and integrity over sensitive medical and research data, allowing diverse contributions without compromising sensitive patient information or workflow stages. This granular control over content and user roles is reminiscent of advanced Notion workspaces.
This transformation is akin to moving from individual, disconnected medical record-keeping systems to a shared, securely integrated digital platform where every team member has authorized access to the most current and validated medical research data, dramatically reducing friction and accelerating initial development of crucial medical insights.
(Image Idea: A split screen showing chaotic document versions of a clinical trial protocol (multiple conflicting edits, redlining) vs. a clean, synchronized collaborative workspace interface with multiple colored cursors, a visible history sidebar showing author timestamps, and clear section ownership for different research roles (e.g., Statistician, Clinician, Biologist). Emphasize secure data flow and network connections within a medical context.)
The traditional peer review system for medical and clinical research, while vital, is plagued by opacity and protracted timelines. Reviewers, often busy clinicians or leading scientists, work in isolation, feedback can be subjective and sometimes non-constructive, and the entire process can take many months, delaying critical drug discovery findings, public health interventions, or new diagnostic methods from reaching the broader community. The lack of transparency can also lead to biases and inhibit constructive dialogue around complex medical issues. This "black box" approach slows down the scientific validation process, creating a significant delay in knowledge flow directly impacting patient care.
An engineered peer review system for medical science would prioritize efficiency, openness, and continuous improvement. Medetary introduces layered, inline peer review models designed to:
How it works (Technical Mechanisms):
This system is built on structured data principles, expert validation, and reputation mechanics, ensuring ethical considerations are paramount. It draws inspiration from how open-source communities review code, but adapted for the nuanced needs of scientific rigor:
Inline & Contextual Commenting Engine with Standardized Annotation: Reviews are not separate documents but are tied directly to specific text, figures of patient data, or statistical analyses within the manuscript. Each comment is a structured data object, allowing for categorization (e.g., "factual error," "statistical query," "methodological clarity," "ethical concern"), tagging, and versioning. This enables "layered" feedback that evolves with the manuscript, providing highly specific input crucial for medical accuracy, much like commenting on code in GitHub pull requests.
Dynamic Credentialing & Medical Expert Reputation System: Beyond traditional affiliations, verified medical professionals on Medetary can build a transparent reputation based on the quality and constructiveness of their past reviews. This involves:
Blockchain-Inspired Verification of Medical Credentials: Cryptographically verifiable attestations for academic degrees, medical licenses, and previous peer review contributions, ensuring reviewer authenticity.
Algorithmic Quality Scoring with Expert Oversight: Anonymized feedback on review quality from authors and other reviewers, aggregated to form a dynamic reputation score. This system is monitored to mitigate gaming and encourage insightful contributions, helping "match manuscripts with the most relevant expertise" (e.g., a cardiologist for a cardiology paper), thereby strengthening the medical review process.
Phased Peer Review Stages with Author and Journal Control: Medetary implements an intentional and layered peer review process, resembling the structured yet flexible review flows in software development:
Private Peer Review (Early, Trusted Feedback): This initial phase allows authors to invite trusted collaborators or chosen experts to provide early, constructive feedback. This feedback is visible only to the authors, enabling them to refine their work privately before broader exposure. This mirrors the internal review processes before a formal submission, akin to a private pre-merge code review.
Journal-Managed Review & Invited Peer Reviewers (Phase Two Expansion): Medetary is evolving to become the direct platform for journals themselves to manage their internal peer review workflow. Journal editors can:
Invite and manage their own pool of peer reviewers directly within the Medetary environment, replacing disparate email and document exchanges.
Facilitate collaborative review discussions among their invited reviewers and editorial teams, all within a structured interface.
Leverage Medetary's granular version tracking and inline commenting for a streamlined, transparent, and auditable internal review process. This replaces external email chains and disparate document versions with a unified, secure system, ensuring every step of the journal's review is tracked, much like how a journal might manage a "forked" version of a submission for internal review.
Public Peer Review (Community Refinement): After a preprint is published, an open, non-anonymous commenting and rating system allows the broader medical and scientific community to contribute. Importantly, public peer reviewers can request to join the review process. If accepted by the corresponding author, they can then add transparent, inline comments that contribute to versioned updates. This creates a clear, traceable audit trail of scientific refinement, demonstrating how community feedback shaped the research, similar to how issues and pull requests drive refinement in open-source projects on GitHub.
This is akin to introducing continuous quality control and iterative testing throughout a medical device's development lifecycle, rather than waiting for a final, potentially flawed product to be released before receiving comprehensive feedback. It ensures that critical medical insights are integrated faster and the scientific record relevant to patient care is continually strengthened.
(Image Idea: A flowchart showing a traditional linear medical journal peer review process (isolated, slow, black box) contrasting with a more agile, circular, and transparent peer review loop. Explicitly show a segment for "Journal Internal Review" with arrows connecting to invited reviewers and editorial teams. Highlight the flow from pre-print to public review stages with icons related to medical data or research papers.)
The medical scientific community produces vast amounts of clinical trial data, public health reports, and basic science discoveries, yet much of it remains locked behind expensive paywalls and subscription fees. This creates significant barriers to accessing crucial medical research findings, particularly for clinicians in underserved areas, researchers in resource-limited countries, or independent medical educators. The result is an inequitable landscape where access to life-saving and health-improving knowledge is determined by financial capacity, not the urgency of public health needs or the drive for scientific advancement. This directly impedes the global advancement and application of evidence-based medicine.
An engineering solution would prioritize universal access and efficient distribution of medical knowledge. Medetary champions:
How it works (Technical Mechanisms):
This is achieved through a multi-faceted approach to secure medical data management and robust interoperability standards, replacing outdated, siloed publication pipelines with a dynamic, versioned ecosystem:
Federated and Decentralized Publishing Options for Medical Artifacts: While Medetary securely hosts manuscripts, the architecture supports publishing to distributed networks, ensuring the persistence and accessibility of medical research. This could involve:
Content Addressing (e.g., IPFS integration) for Research Data: Scientific artifacts, including de-identified patient datasets, omics data, or imaging results, can be assigned unique, content-based hashes. This ensures their integrity and enables decentralized retrieval from various nodes, making "instant preprint sharing" resilient to censorship or single points of failure, crucial for widespread medical data availability.
Standardized Metadata for Clinical Discoverability (FAIR principles): Rigorous application of FAIR principles (Findable, Accessible, Interoperable, Reusable) means every published medical artifact comes with rich, machine-readable metadata (e.g., schema.org, ICMJE guidelines, HL7 FHIR standards for health data extensions). This ensures global discoverability through specialized medical search engines and aggregators, regardless of the hosting location.
Standardized API Integrations for One-Click Submission to Medical Journals: "One-click submissions to partner journals" are not magic; they are built on robust, secure API integrations. Medetary develops:
Common Data Models for Medical Manuscripts: Manuscripts and associated data are structured using industry-standard medical publishing formats (e.g., JATS XML, CRediT taxonomy for contributions, PRISMA guidelines for systematic reviews).
Automated Validation Engines for Clinical Compliance: Before submission, a system validates the manuscript against journal-specific medical requirements (e.g., formatting for figures showing patient results, reference styles, word counts, CONSORT statement adherence for trials), minimizing rejections due to trivial errors and accelerating the path to publication.
Direct API Handshakes with Medical Journal Submission Systems: Secure API connections with journal submission systems allow for programmatic transfer of the validated manuscript and metadata, eliminating manual uploads and reformatting. This makes the submission process akin to a secure, automated data transfer protocol, directly to platforms like PubMed Central or other clinical registries.
This approach is about ensuring that the blueprints and findings relevant to human health are readily available for everyone to build upon, not confined behind proprietary walls. It ensures that valuable medical research insights can flow freely, maximizing their potential impact on global health and innovation.
(Image Idea: A world map with light radiating outwards from medical research hubs to all corners, symbolizing truly global, barrier-free medical knowledge dissemination. Show interconnected nodes representing decentralized data sharing (with health data icons) and direct API pathways to specialized medical journals/databases.)
The existing medical research publishing infrastructure relies heavily on manual processes for everything from formatting and submission to data management. These manual steps are time-consuming, prone to human error, and can introduce inconsistencies. More fundamentally, concerns around the reproducibility of clinical findings, the integrity of preclinical data, and the transparency of methodological procedures persist. A lack of standardized methods for verifying data and experimental procedures undermines the very foundation of scientific trust in medicine, which is paramount for patient safety and effective treatment.
Modern engineering principles offer powerful solutions to enhance both efficiency and trustworthiness specifically in medical research:
How it works (Technical Mechanisms):
This involves deep integration of computational validation and immutable record-keeping, with a focus on HIPAA compliance and data security. It transitions from a static publication model to a "living space for scientific thinking," where continuous progress is visible:
Intelligent Validation & Compliance Engines: Medetary employs rule-based systems and pattern recognition to ensure adherence to scientific and ethical standards:
Automated Compliance Checks for Clinical Studies: The platform can scan manuscripts for adherence to ethical guidelines (e.g., IRB approvals), funding agency requirements (e.g., NIH policies), and potential conflicts of interest specific to medical practitioners or pharmaceutical companies.
Plagiarism Detection & Originality Scoring for Medical Texts: Advanced algorithms analyze text against vast databases of medical publications to ensure originality, critical for maintaining academic integrity.
Clinical Data Integrity Pre-flight Checks: For submitted de-identified patient datasets, genomic sequences, or imaging reports, automated checks can flag inconsistencies, missing values, or potential anomalies based on predefined validation rules and statistical patterns, minimizing errors before review.
Cryptographic Data Provenance & Version Control for Medical Research: This is Medetary’s core commitment to "reproducibility by design" in the medical field:
Immutable Content Hashes for Clinical Artifacts: Every version of a medical manuscript, raw patient dataset, analysis script, or clinical trial protocol is fingerprinted with a unique cryptographic hash (e.g., SHA-256). This hash is then timestamped and potentially anchored to an immutable public ledger (like a blockchain or a verifiable timestamping service). Any alteration to the original content would change its hash, immediately signaling tampering and preserving the integrity of medical evidence.
Verifiably Linked Artifacts for Comprehensive Clinical Context: Manuscripts are programmatically linked to their raw data, analysis scripts, and experimental protocols. These links are not just URLs; they are verifiably persistent references within the platform, ensuring "every version, comment, contribution, and dataset is traceable," crucial for auditing clinical research trails.
Reproducibility Environment Specification for Computational Biology & Bioinformatics: For computational medical research (e.g., genomic analysis, drug-target prediction models), Medetary's platform can capture and specify the exact software environment (e.g., specific versions of R/Python libraries, dependencies, operating system images) used to generate results. This can be achieved through containerization technologies (like Docker), allowing other researchers and regulatory bodies to re-run analyses with identical conditions, thereby verifying computational medical findings.
This is about creating a system where scientific rigor and reproducibility are inherent to the process, not an afterthought, and where trust in medical research data is built directly into its digital infrastructure. It builds trust by making every step transparent and verifiable, leveraging technology to enhance, rather than complicate, the scientific method for the ultimate benefit of patient care.
(Image Idea: An abstract representation of secure medical data flowing seamlessly and securely, with icons of automated validation (e.g., a checklist, a magnifying glass over data, a shield for security), alongside chain links or hash symbols demonstrating data immutability and verifiable provenance for clinical trials. Emphasize security and precision.)
At Medetary, we are not merely observing the inefficiencies in medical research publishing; we are actively engineering a solution from the ground up. Our platform is meticulously designed with the medical researcher and clinician in mind, aiming to disrupt the status quo by:
Elevating Medical Research: Providing intuitive tools and a streamlined environment that facilitates the entire medical research lifecycle, from initial hypothesis generation to global dissemination of findings.
Fostering Medical Collaboration: Offering secure, real-time workspaces that enable seamless, global co-authorship among diverse medical specialties and interdisciplinary teamwork.
Promoting Transparency: Implementing open peer review models and robust version tracking that build trust and accountability into every medical scientific endeavor.
Accelerating Dissemination: Ensuring rapid knowledge sharing through instant preprint options and simplified, one-click submissions to reputable medical journals and clinical databases.
Just as engineers design robust, scalable, and reliable infrastructure for other critical sectors like transportation, energy, or communication, we believe the medical research community deserves a publishing infrastructure that is fit for the 21st century. One that prioritizes speed, transparency, accessibility, and the advancement of knowledge for the benefit of all humanity's health.
The frustrations are a clear call to action for innovation. It’s time for an engineering mindset to take center stage in reshaping medical research publishing. We invite researchers, clinicians, funders, and healthcare institutions to join us in building a better future for scientific discovery – one where bridges of knowledge are constantly being built, not blocked by unnecessary barriers.
Stay tuned for updates on Medetary's progress towards our Beta Access in Q4 2025!